The latest release (21R1) of updates to Veeva CRM and Veeva Vault rolled out for general availability on 8th & 16th April 2021 respectively. As with most releases from Veeva, 21R1 contains a vast number of feature enhancements, adjustments and fixes, which on this occasion centre predominantly around Veeva Vault & Promomats, although there are a few key enhancements to the CRM.
To save you time, we’ve picked out the highest impact updates for CRM/CLM optimisation and effectiveness.
Key DSA Effectiveness Updates From 21R1
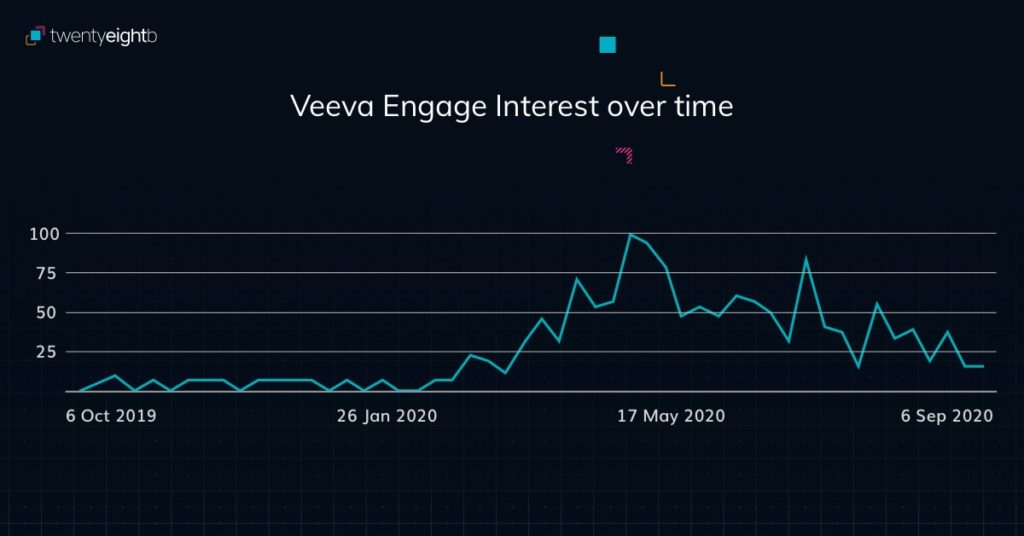
CRM Users Attending an Engage Meeting
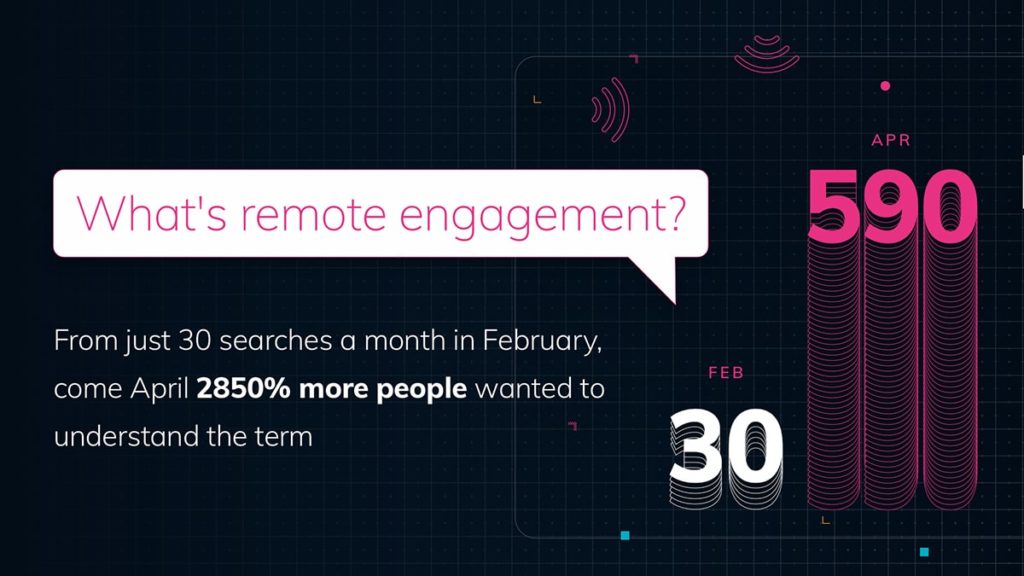
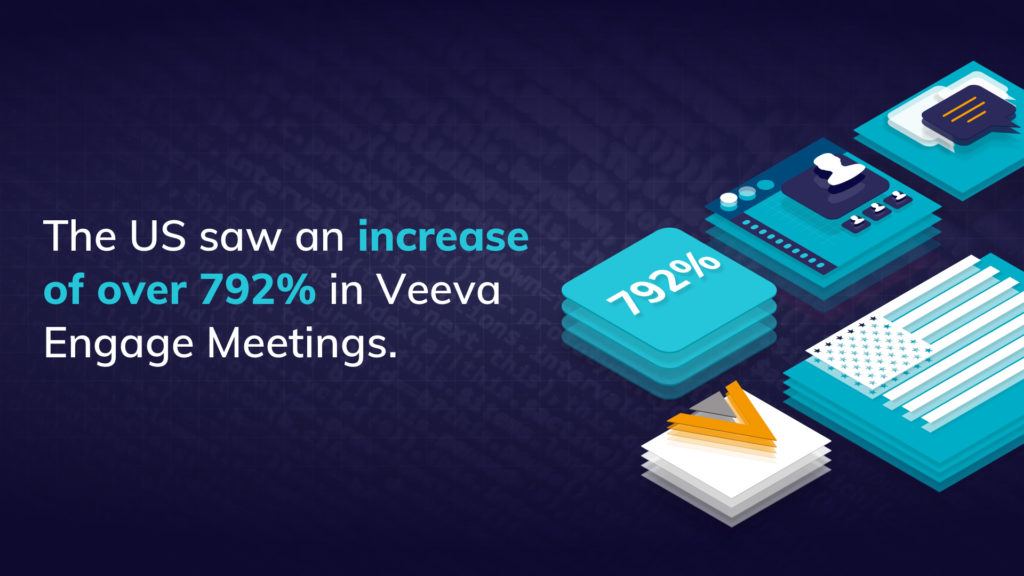
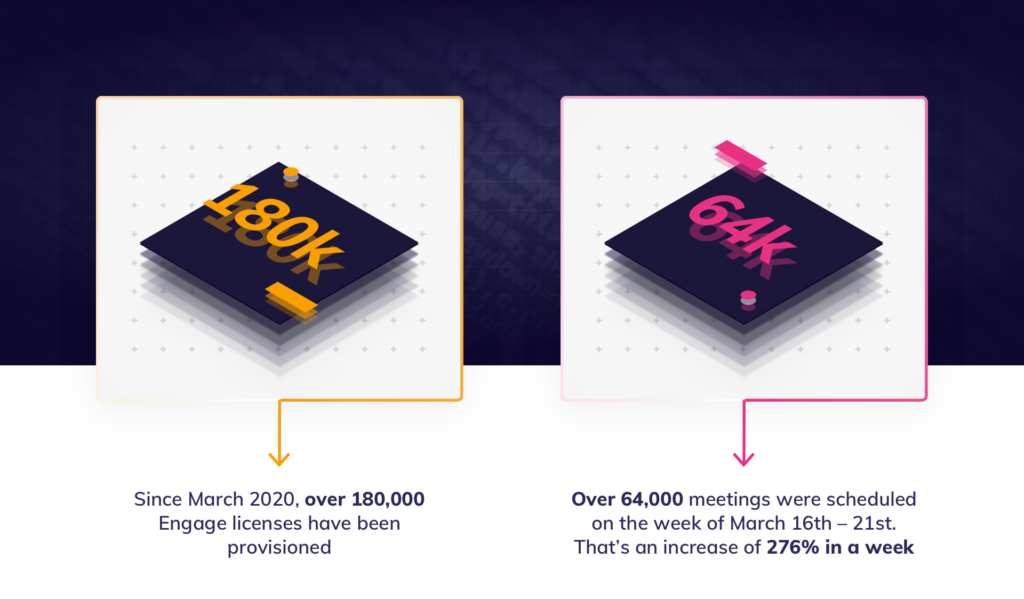
With face-to-face meetings having made way for remote presentations, it has become more challenging for field team managers to observe meetings or deliver training. Joining virtual meetings on Veeva CRM Engage Meeting had been a hassle and required a workaround, so Veeva’s update to allow meeting hosts to invite any CRM users to join remote meetings will be very welcome.
Video Playback Speed Controller
As a result of the huge shift to remote presentations, video has become a more effective sales tool than ever before. However, rich media without sufficient control for the viewer can cause UX friction and frustration.
Fortunately, this latest update – when enabled – gives the HCP greater control over the presentation they are viewing, by being able to speed up or slow down video that plays within Vault. The handy +/- buttons within the player make it easy to toggle all the way from 0.25x normal speed right up to 2x. It is worth noting that this functionality isn’t currently supported on Android devices.
MyInsights Studio
Generating actionable insights from data gathered in the field is a huge area of opportunity for brand and sales team leaders, so Veeva’s move to open up MyInsights dashboard creation to non-developers could be a significant step forwards. Access to the new MyInsights studio can be requested by a support ticket and will mean that companies can begin to create their own interactive visualisations and custom pages for direct deployment to Veeva CRM. This is a chance to ‘dip a toe in the water’ of the huge opportunities that exist within MyInsights, which may well in turn encourage greater interest in the more powerful selling effectiveness dashboards that we have seen prove so valuable to some of our clients.
Modular Content: Create & Approve Modules
A term that we at twentyeightb champion as being at the heart of efficiency creation within content management, ‘modular content’ continues to evolve as a concept within the Veeva ecosystem. This latest release is a further step in the right direction; providing users with the framework to create and approve content modules, which can then in turn be reused in multiple material pieces.
This centralised control over Claims Library items, image documents, and data assets and the rulesets that can be applied to them promises significant time-savings if effectively adopted. We believe there’s a lot more to come from Veeva around modular content, so sign up to our newsletter to get key updates throughout the year ahead.
What Else Changed In 21R1?
For those more involved in compliance, CRM and asset management there were a handful of other updates of real note.
Rich Text Fields for Vault Objects
While formatting options are commonly known and used within Veeva, the 21R1 update sees these options made available to format the content of field values. The configurability of formats including text alignment, font size and font family can now be used by Admins across text fields to configure Rich Text, such as:
- Object list pages
- Object detail pages
- Reporting
- Workflows
- VQL
- Vault Loader
- Exports
- APIs
Auditing and compliance may determine that information needs to be captured in a specific format, so the above change provides additional opportunity for you to ensure that content entered in text fields is stored in the exact format required – saving on admin and compliance headaches.
Video Review: Bringing Forward Annotations
The video approvals process has also been made a little bit easier with this release. Annotations of amends or updates can now be brought forward on video documents to the most recent version of the document. This removes some friction around version management in a format whereby amends can be costly and time consuming to make, so carefully tracking changes can really make a difference.
Multi-Document Workflow: Edit Document Fields
Another positive move towards streamlining the content production process, this UI update makes it easier for users to make changes to document fields within the multi-document workflow viewer. With the process of selecting/ deselecting various files within a batch having been simplified, users should experience efficiencies while also reducing the risk of manual errors.
Web Tab URL Tokens
This update brings with it the ability to integrate an external web application with Vault, through some basic cross-interaction via query string. This provides the benefit of being able to convey content that sits outside of Vault in a controlled and regulated manner, that also doesn’t inhibit the user journey.
If you’re interested in reading the full list of updates included within the 21R1 release, you can find the CRM release notes here and the Vault release notes here. As ever, if you’d like advice on how to make best use of any of the new features in order to better optimise your use of Veeva, then please get in touch with our expert team.