Control has often been a key phrase in pharma, with regulations determining the way in which pharmaceutical companies and their reps can – and more importantly, cannot – communicate with healthcare professionals (HCPs) and the general public. But where does a modular approach come into this?
What is modular content?
Modular content essentially describes the method of developing components that can be reused and dynamically update – in turn providing centralised control over content could exist in multiple locations and contexts.
In web development circles, the concept of modular content is fairly tried and tested. Borne out of a desire to reduce development overhead in updating multiple instances of similar components, it is embedded in a lot of the best practice around website development on popular content management systems (CMS’s), such as WordPress, Drupal and Joomla!.
In pharma terms, modular content has typically been framed slightly differently. While website developers may use the approach primarily as a means of economising effort, software and systems developers in pharma would adopt modular content primarily as a means of control.
How can pharma adopt a modular approach?
In some respects pharma brand teams and agencies may already be working in a modular way, but without really realising. Veeva talk about ‘modular response content’ that is managed from Veeva Vault MedComms and how it provides a central point of truth. When harnessed correctly, this technology streamlines operations, centralises information and provides greater opportunity for data collection and analysis.
Modular content in pharma email communications
Features within Veeva Approved Email are constructed and inputted using a modular framework. The software admins retain control of the content, providing it to the rep as list of approved messages to choose from. Crucially, this ensures that the communication shared with HCPs is moderated and – as the name states – approved. These modules are referred to as ‘fragments’ within individual emails, as shown in the example below.
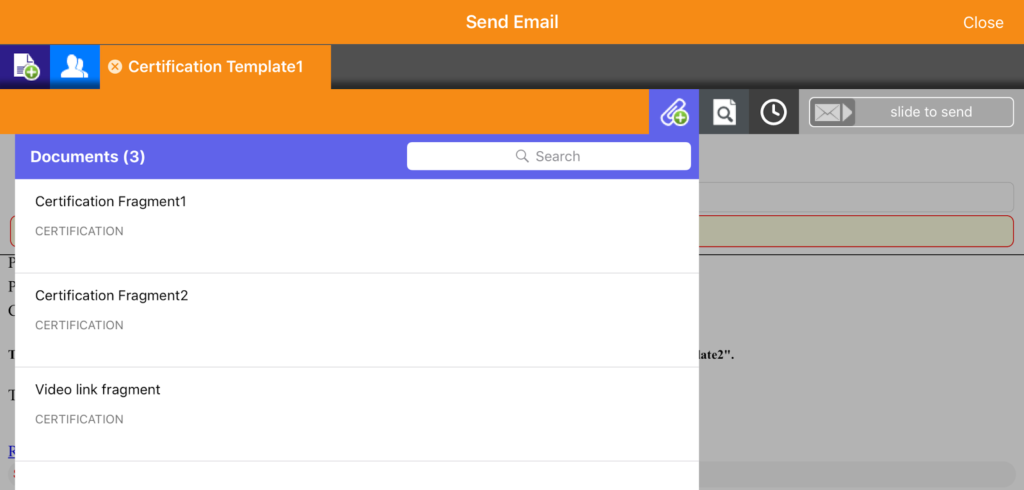
To get a better understanding of how to use Veeva Approved Email for better sales outcomes, read our VAE introductory guide or watch the first in our series of video tutorials on VAE best practice.
Modular content in CLM presentations
Customisation of content is a key factor in CLM effectiveness and modular content puts more options in the hands of the rep, without compromising compliance. By having the option to drag and drop different graphical elements, charts and diagrams into a dynamic edetailer, pharma sales reps can take greater control of the narrative that they feel will influence the decision of a HCP.
Make sure you plan for modular content
For modular publishing to work, a thorough upfront assessment of requirements and a detailed specification that covers every eventuality needs to be produced. Planning for every instance where modular content may be required and then inputting the content that can display within those fields should set you on the right footing – however there is no substitute for consulting with Veeva specialists who understand the nuances of this incredibly powerful platform.



