Welcome to our Veeva Insights Video Series, the series that is designed to help brand teams and creative agencies better understand and leverage the tools available to them on the Veeva CRM approved platform.
Within this first episode, James Harper, Founder and Managing Director of twentyeightb, gives you an insight into Veeva CRM Approved Email, and guides you through using it within the CRM for iPad app, previously known as iRep.
First things first, what is Veeva CRM Approved Email?
Veeva CRM Approved Email enables field teams to send compliant, approved, personalised content to customers and contacts via email. It’s an integral part of the Veeva ecosystem, meaning it’s easy to use and it works with other CRM/CLM tools and content such as detail aids, Veeva engage and vault documents.
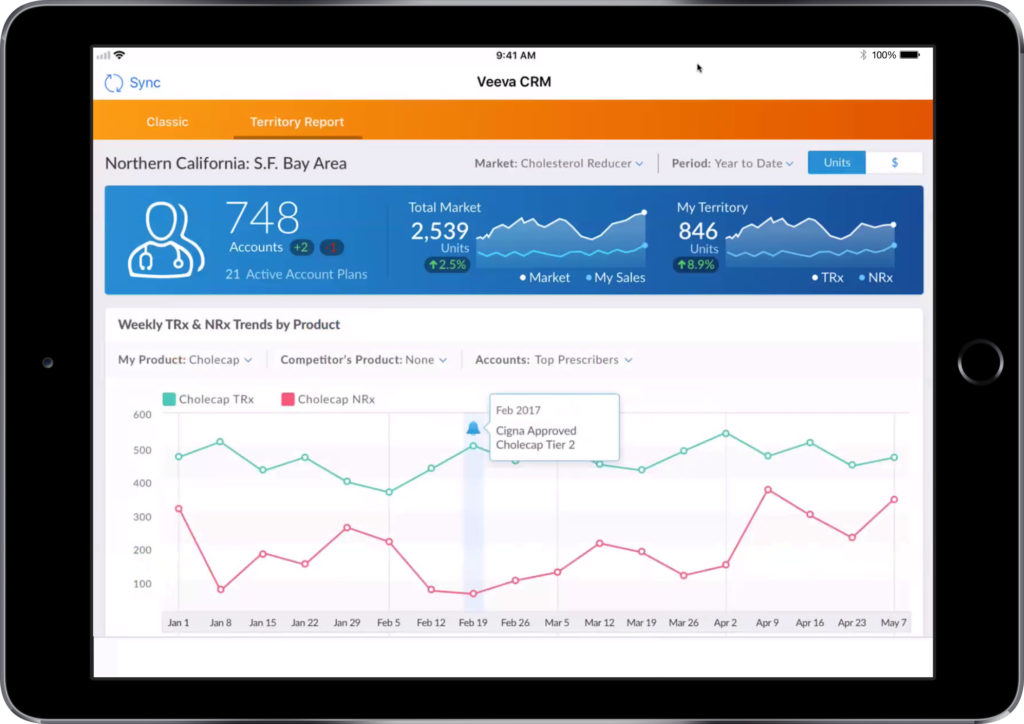
Most importantly, Veeva’s usage analytics feed back into the CRM system, providing you with detailed information on your email’s send and open rates. Not only can these analytics be used by you to motivate field teams, increase usage, sales and enhance customer engagement, but they can potentially provide you with invaluable market insight.
Have a watch of the first episode below, or read our key takeaways and find out more about Episode 2 of our Veeva Insights Video Series, coming soon.
Key takeaways:
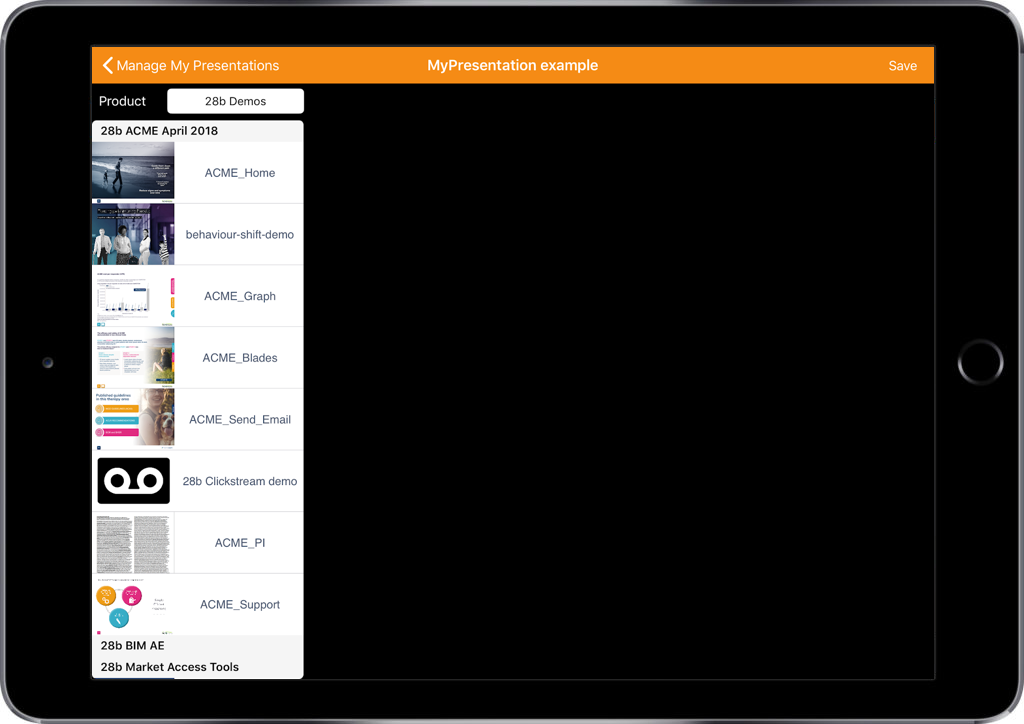
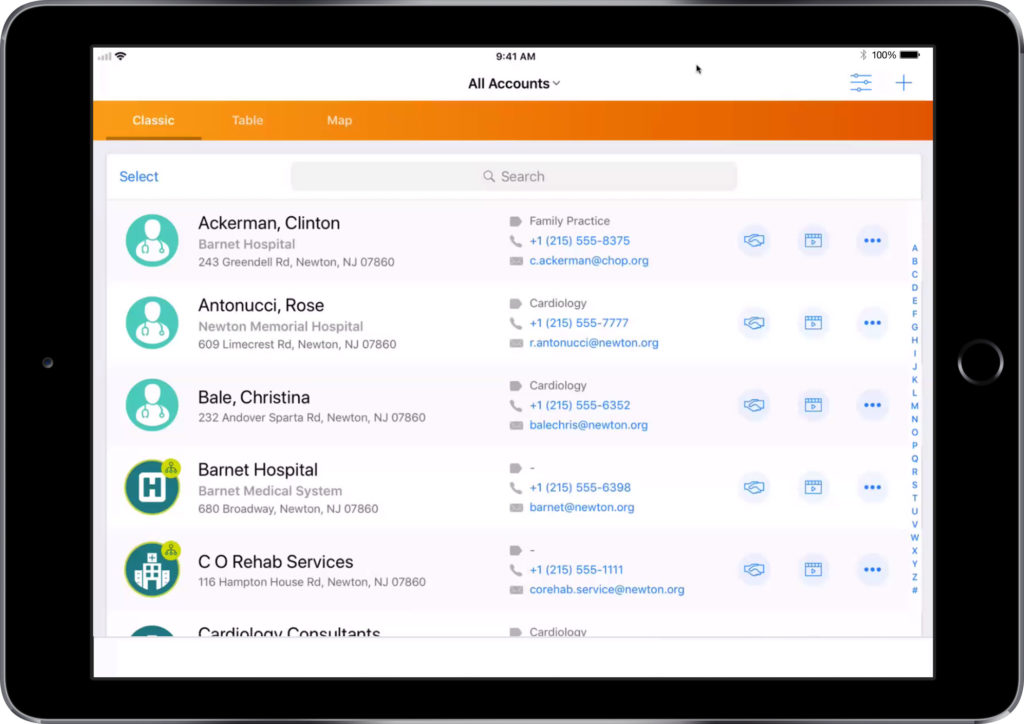
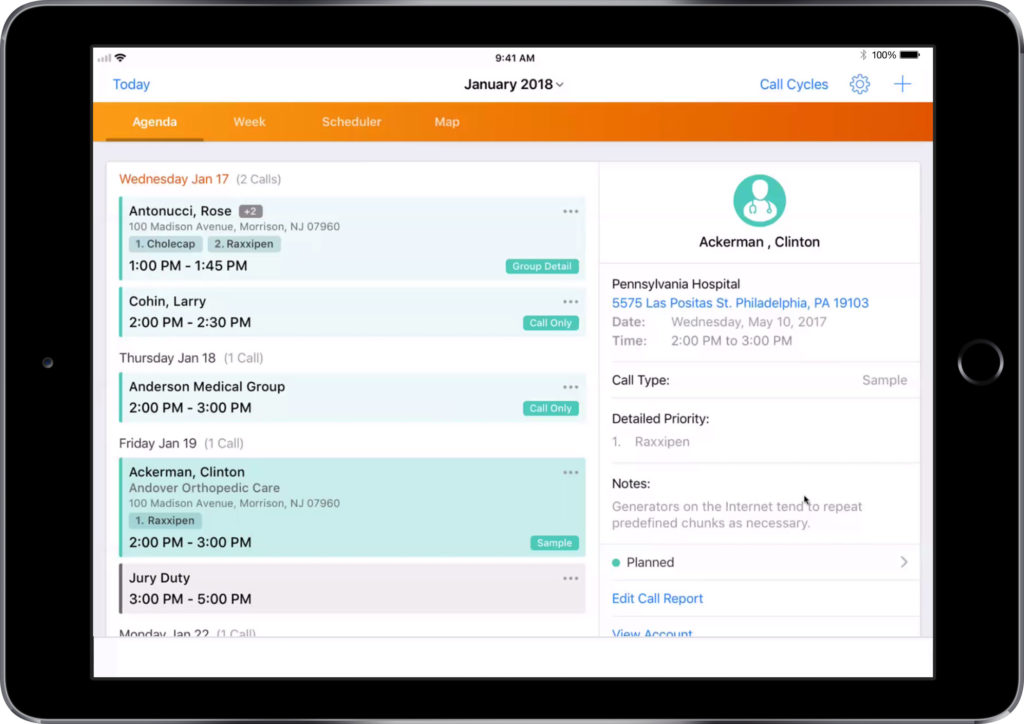
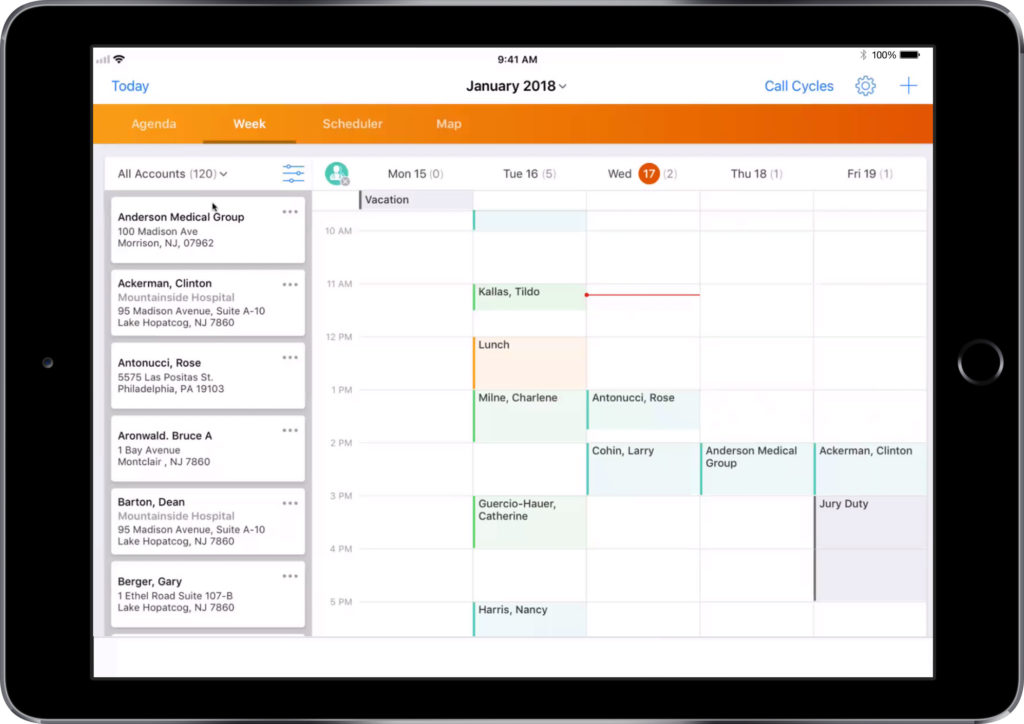
Selecting a customer and template for your email.
(01:53 – 02:26)
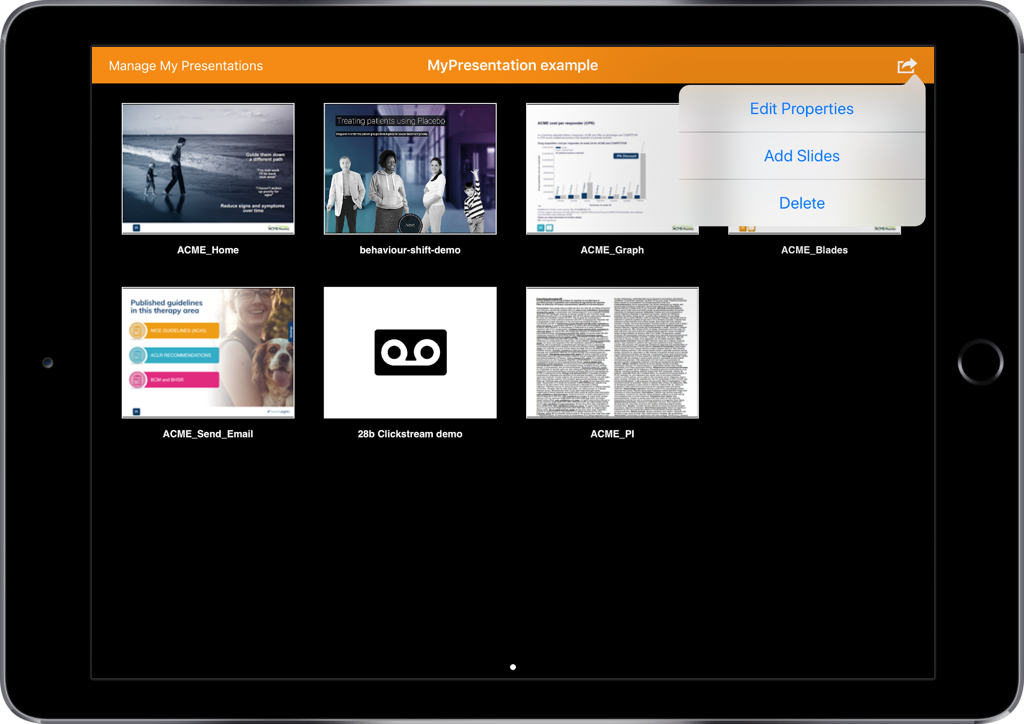
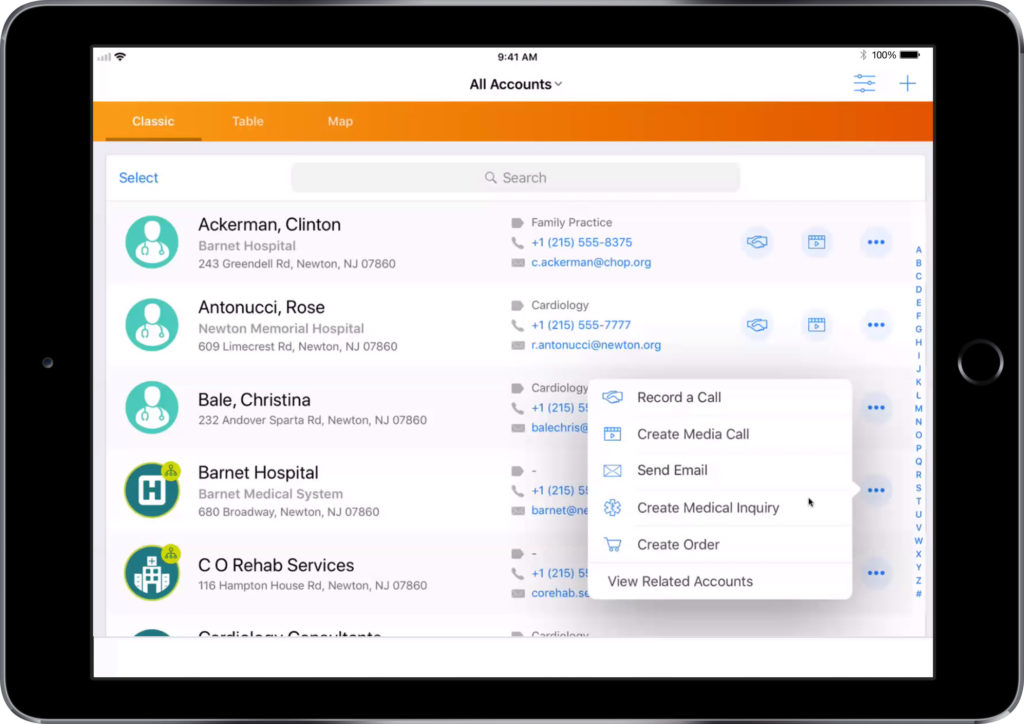
- Select your target customer from the list
- Using the three dots on the top right, select ‘Send Email’
- Select a template from the list provided
As you would expect, Veeva CRM Approved Email offers a highly granular level of control in terms of what email templates and content are available to users. This helps to ensure that only the content that is appropriate and approved by you will be shown to your customer.
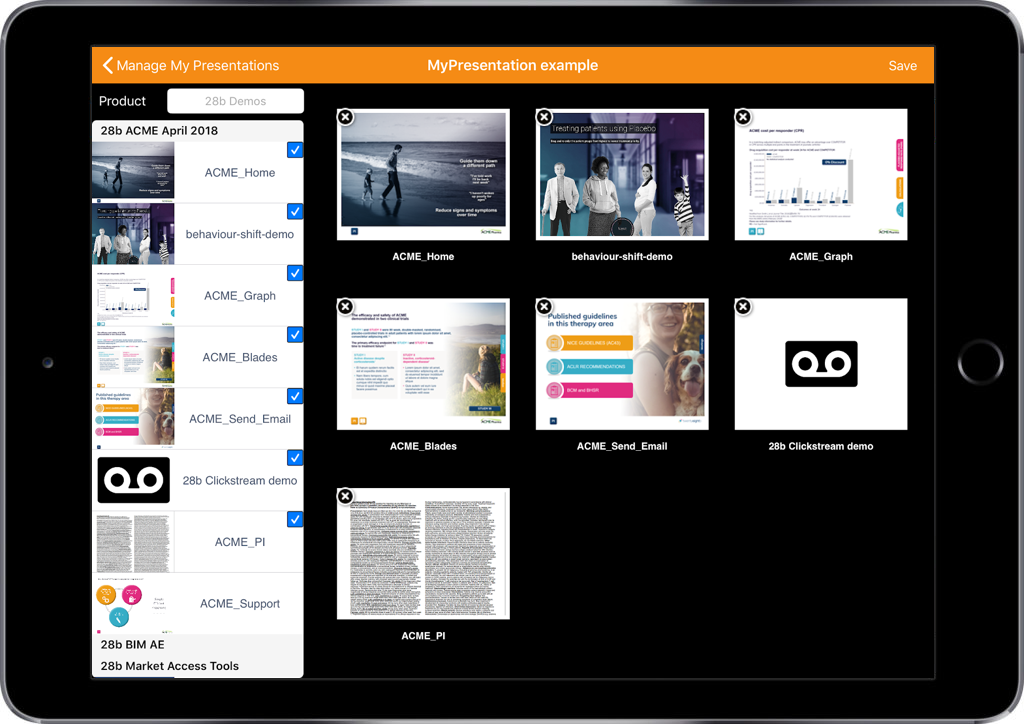
Personalising your email content and adding fragments.
(02:27 – 03:44)
- Personalise your content within the template
- Select the paperclip icon to access fragments
- Select the fragments relevant to this email
- Re-order fragments within the template
By using Veeva CRM Approved Email you give the user the ability to personalise the content within an email template through drop down lists and free text fields. You can also blacklist words and terms, as well as mandate the completion of a particular field.
Once you have selected the fragments that are relevant to your customer you have the ability to re-order them within the template. The references will then re-number to match, thanks to an automated referencing system.
Previewing and sending your email.
(03:12 – 04:23)
- Select the eye icon to preview the email prior to sending
- Return to editing by selecting the pencil icon
- Send your email using the slider!
From selecting a customer right through to sending an email, Veeva have included intuitive touches to ensure the user feels comfortable with the interface, and comfortable using it to communicate with their customers.
In the next episode:
In Episode 2, we take a look at how a Veeva CRM Approved Email can be launched directly from within an eDetail, plus, how you can use analytics and a MyInsights dashboard to inform and enhance a user’s effectiveness in the field.
Have a question that wasn’t covered in our first episode? Get in touch with a member of our team using our quick question form.