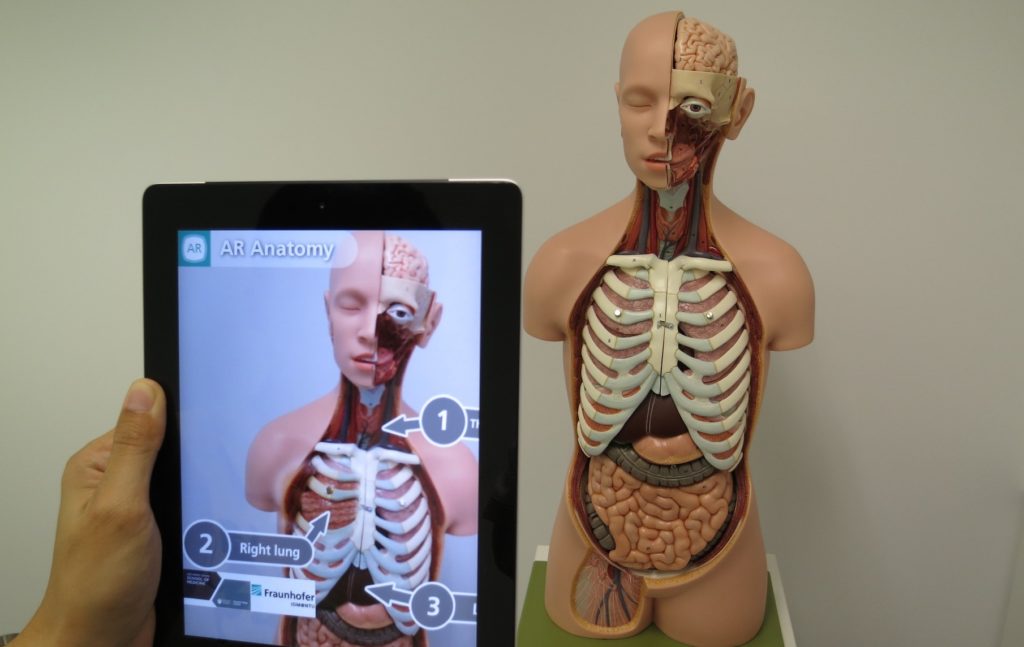
The second article in a series of three on harnessing a data-driven digital strategy, this piece from Nick Woolnough looks at how opportunism in content marketing can be just as powerful – if not more so – than the considered outputs of the traditional ‘brainstorming’ or planning sessions that are the mainstay of content strategy.
Based on a talk that Nick – Head of Marketing Operations at StrategiQ – gave at the Pharma Multichannel Marketing Meetup in December 2018, this series looks at the concepts and approaches applied in mainstream marketing that can be adapted and moulded to fit the pharma sector.
“It’s not all creativity clubs, workshops & brainstorming”

There’s a bit of a misconception that many hands can make light work when it comes to content ideation (the process of conjuring up and refining content ideas). The thought of gathering people from different areas of the business together in a boardroom with a whiteboard and a marker pen to brainstorm feels collaborative and diverse, but it still doesn’t directly address the main purpose of content marketing as a principle.
To be useful and therefore successful, content needs to directly address the needs of the target audience and have a means for getting in front of that audience.
A room full of experienced people in their field will likely come up with some great ideas, but they will be ideas restricted to their wingspan of knowledge. Is it still current? Is the competition too great? This is research and ideally data that will have to be backfilled to validate the ideas. When you start with data, the opinions of the six people in the boardroom become less important, their time can be saved and the actual needs of the target audience can be addressed with relevant content. Effective content needs to plug a gap, not add to the white noise around a subject area.
Find it, fill it
While not alone in any respect, the pharma industry can be guilty of publishing content to the web that fails to give consideration to what search engines – and their users – want and need. To be useful, digital content needs to ultimately be the answer to a question. While the way in which we frame our search queries to Google has changed as the intuitiveness of its algorithms continues to evolve, they are still ‘queries’. Ask Jeeves may not be the search engine of choice these days but the common principles still remain in that we request information and a search engine returns the most relevant results.
You don’t have to be an SEO ‘Guru’ or ‘Ninja’ to find opportunities to generate search engine traffic, in fact a member of our team at StrategiQ wrote a detailed blog on how you can use tools to hunt down opportunities. A few key points though:
Find a niche within a niche
If your businesses niche is pharmaceutical device manufacturing, don’t saturate your website with thematically similar content on device manufacture. Dig into the detail and expand on the detail – the people you want to talk to online are likely to already understand what you do and be craving more depth. Cover a particular component of a device in depth or a patent – whatever it is, sense check it against the search results for relevant terms to see whether it has been done to death by competitors. If it has, then make sure your content is more detailed and better structured. If it hasn’t, great!
Hunt down the PDFs
The pharma industry is chock full of whitepapers and other pieces of content that have been consigned to PDFs. Google doesn’t ignore PDFs in the way that some may suggest, however it will naturally prioritise content on webpages as they are easier to crawl and index and provide a continuation of the user experience, rather than an isolated document. Have a look at detailed search queries that you may want to target (such as the below example) and find opportunities to publish content that others have covered in a PDF – in these search verticals you’ll have a higher chance of success.
Don’t wait
This one can be tricky in pharma, but if you can launch some content earlier than the competition, regardless of whether you have access to all the information you need, you can secure prominent Google rankings. The timeliness of content is key – there is no harm in pushing something out as a placeholder and then evolving it over time – Google loves content that has been refreshed and updated.
Think about your end game
What role do you want content to play not just in your marketing strategy, but also your business strategy? Content and social media marketing can be perceived as being light years away from the coalface of a business; directly generating revenue or converting customers. In some respects that is true, but both are great tools for nurture, particularly in B2B sectors.
Consider what you want people to find you business for or associate your business with. It’s important to accept that these people won’t always be your potential customers or end users, but are relevant enough that they may share your content or your brand message to others that might be. By broadening your target audience for content beyond your typical target audience for acquisition, you may well find that the overall impact on your brand is far stronger.
The next article in this three-part series will explore the user experience principles that can be adopted by keeping an eye on the silent majority of website visitors. Sign up for the twentyeightb newsletter to keep yourself abreast of all our latest content.